| Author | Message | ||
David Day (daverday) New member Username: daverday Post Number: 2 Registered: 11-2009 |
Karl, I was told the spectrum on the web was the power spectrum from our downward looking spectrometer (the one with the 1 degree field of view pointed right at the plume) averaged over all scans from about 20 seconds after the centaur impact to 60 seconds after the impact divided by an equivalent number of averaged scans just before the impact. So this would be like Reflectance if there were source light to be reflected. The original intention was for sun light coming in nearly parallel to the moon's surface (at the south pole) to scatter off the cloud and be collected by our spectrometer. I would have expected to see the black body curve of the sun with dips where absorbers were. (This is what was seen when the spectrometer was pointed at earth a month before impact). So in the spectrum in question we see a black body curve of a couple hundred degrees (relative to the pre-centaur impact power spectrum where I heard in the press conference they were estimating the surface at -230C). This suggests to me that light received was not from the sun but possibly the black body radiation from the hot cloud (possibly still below the crater rim and not out in the sun light- I'm guessing at this). One thing to consider is that there may both "hot" zones in the cloud and very "cold" zones in the cloud... material at the far edges of the crater, ice, may remain ice in the plume, while right at the impact point everything would be hot and vaporized. By the way, our spectrometers were run in "Flash" mode for a short period during the centaur impact where instead of taking full spectra (normally about 700 milliseconds per scan) it took data at six different wavelength regions for a very crude scan taking about 12 milliseconds. The purpose of this was to collect the emission data from the plasma "flash" which had a very short duration (like a couple hundred milliseconds). The spectrometers successfully recorded the flash but we must wait for Nasa to report more on that. But just to be clear, the "flash" data was not the data shown at the press conference. David | ||
Karl Norris (knnirs) Advanced Member Username: knnirs Post Number: 21 Registered: 8-2009 |
David, I respect your right to not comment, but can you tell us whether the spectra presented to the public are emission spectra or transmission spectra. It appears these spectra were from the Polychromix spectrometer. Karl | ||
David Day (daverday) New member Username: daverday Post Number: 1 Registered: 11-2009 |
All, I have been silent on this discussion and will remain mostly so, however, I did send Tony Colaprete an email inquiring about plans to publish their analysis. His response: "We'll be giving another presentation at the American Geophysical Union meeting in SF in December and then again in March at LPSC in Houston. We plan on submitting initial papers to Science in about two weeks." David | ||
Lois Weyer (lois_weyer) Senior Member Username: lois_weyer Post Number: 33 Registered: 7-2002 |
There are a couple of relevant references to the hydroxyl absorption in my and Jerry's book, in Chapter 6. I can send the chapter to you if you send your email address to my email address. Mine is [email protected]. | ||
Howard Mark (hlmark) Senior Member Username: hlmark Post Number: 297 Registered: 9-2001 |
Karl - if Lois has a reference, so much the better, but we know that the 1940 band is a combination band of the -OH stretch and the -OH bending vibrations. A free OH ion (note that there is no bond indicated on the oxygen) id s diatomic species that can only stetch and rotate, but it can't bend, therefore there is no bending vibration and no combination band that includes the bending vibration. I was also going to question Lois on that, until I realized the situation. \o/ /_\ | ||
Karl Norris (knnirs) Intermediate Member Username: knnirs Post Number: 20 Registered: 8-2009 |
Lois, This is new to me. Is there a reference? Karl | ||
Lois Weyer (lois_weyer) Senior Member Username: lois_weyer Post Number: 32 Registered: 7-2002 |
I would like to remind everyone that the hydroxyl ion has a peak at about 1420 nm, but would not have anything at 1940. So, if the "water" is in an ionic state, wouldn't that explain the dip at 1420, but not at 1940? | ||
Karl Norris (knnirs) Intermediate Member Username: knnirs Post Number: 17 Registered: 8-2009 |
Revised spectra. I am still convinced that the spectra shown in the 11/13/2009 NASA news release are emission spectra from the plume, so I have modified my spectra of water vapor, liquid water, and ice to include the relation to a blackbody reference at 230 C. Again these spectra are scaled for plotting, and do not represent a stated amount of water, but should represent the expected shape of the different absorbers. I am now concerned as to my responsibility as a citizen, when I suspect that NASA scientists may be wrong in their interpretation of low quality spectra. Any thoughts from other U.S. citizens. Karl Karl 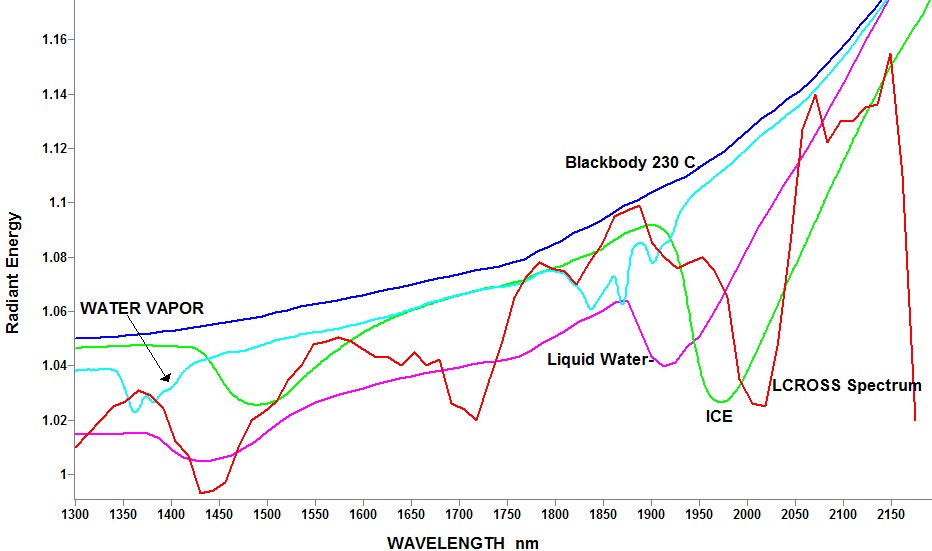 | ||
Frederick G. Haibach (fhaibach) New member Username: fhaibach Post Number: 1 Registered: 6-2009 |
All, I�ve been following the LCROSS mission since we were first asked to adapt our �commercial, off-the-shelf� spectrometers for the mission. LCROSS was formed out of the extra payload capacity for the LRO mission, as such it was designed as a cheap, disposable mission. Critical to mission success was ability of our spectrometers to meet the mission specifications: survive launch and space; low cost, power and weight. Dave Day did receive partial data from the mission. NASA was providing this to Dave to confirm that the instruments were performing correctly. We should not divulge that data without NASA�s approval. I think that all of you will agree that divulging customer data without prior approval is a poor policy. The requested LCROSS NIR data and mission details should come from NASA directly. Most data can be requested by amateurs and researchers from the National Space Science Data Center. http://nssdc.gsfc.nasa.gov/about/about_nmc.html For access to some data, a proposal or confidentiality agreement may be required by the mission director. I made such a request in the late 90�s and my hard disk was overwhelmed by the volume of data. As with most researchers, NASA will probably reserve the right to release data after NASA scientists have had a chance to publish their findings. Unlike some US government agencies, NASA is remarkably open with its data and findings, although all US government agencies have the same mandate to release research data for projects that do not contain restricted information. In general, I�ve found that the quality of research in the remote sensing community to be very competent. They do an excellent job of characterizing spectrometers and the algorithms used to interpret the data are worthwhile looking through. Some of them provide innovative solutions for more, ahem, �grounded� applications of NIR. Sincerely, FgH... Frederick G. Haibach, Ph.D. Dir. Appl. Eng., Polychromix, Inc. | ||
Tony Davies (td) Moderator Username: td Post Number: 218 Registered: 1-2001 |
Howard, I think they go to the Large Hadron Collider! Tony | ||
Howard Mark (hlmark) Senior Member Username: hlmark Post Number: 296 Registered: 9-2001 |
Tony - HOW good the simulation is I don't know, but they wouldn't commit billions of dollars without SOME foreknowledge. Large vacuum: probably. Moon rocks: I dunno. But knowledge about the moon rocks: yes. After all, they've been studying them for decades. Then they could use that knowledge to get earth rocks that were alike. About that last part: don't be so sure. Do you know where every penny of your taxes go? \o/ /_\ | ||
Tony Davies (td) Moderator Username: td Post Number: 217 Registered: 1-2001 |
Hi Howard Do they have access to a very large vacuum? Do they have any rocks from craters near the south pole? You are I'm not! Tony | ||
Howard Mark (hlmark) Senior Member Username: hlmark Post Number: 295 Registered: 9-2001 |
Tony - from what I understand about the way NASA works, and approaches these sorts of problems, before they commit to sending a probe, they simulate on earth, as best they can, the situations they expect the probe will encounter. In this case, for example, before they sent the LCROSS, I would guess that they had done several crashes of a piece of metal the size, shape and general composition of the impactor, onto pieces of rock that were as nearly the same as they expected to be present on the moon, at the site. NASA isn't limited to COMPUTER simulations; they can do physical simulations, too; after all they're not paying for it, we are! Thus, from these simulations, they would have a fairly good idea, before the rocket ever took off, how the rock would shatter, and how much dust would be produced, and the particle size distribution of the dust. And from that, an estimate of the photon pathlength. \o/ /_\ | ||
Tony Davies (td) Moderator Username: td Post Number: 216 Registered: 1-2001 |
Hi Moonies! I just found this on http://en.wikipedia.org/wiki/Lunar_water The search for lunar ice continued with NASA's Lunar Reconnaissance Orbiter (LRO) mission, launched June 18, 2009. LRO's onboard instruments carried out a variety of observations that may provide further evidence of water. On October 9, 2009, the Centaur upper stage of its Atlas V carrier rocket was directed to impact Cabeus crater at 11:31 UTC, followed shortly by the LCROSS spacecraft itself that flew into the ejecta plume and attempted to detect the presence of water vapor in the debris cloud.[29] Although no immediate spectacular plume was seen, time was needed to analyze the spectrometry data. On November 13, 2009 NASA reported that after analysis of the data obtained from the ejecta plume, the spectral signature of water had been confirmed.[1] However, what was actually detected was the chemical group hydroxyl ( � OH), which is suspected to be from water,[3] but could also be hydrates, which are inorganic salts containing chemically-bound water molecules. The nature, concentration and distribution of this material requires further analysis.[1] The amount of water discovered is put in perspective by this comment from Robert Zubrin: "The 30 m crater ejected by the probe contained 10 million kilograms of regolith. Within this ejecta, an estimated 100 kg of water was detected. That represents a proportion of 10 parts per million, which is a lower water concentration than that found in the soil of the driest deserts of the Earth. In contrast, we have found continent sized regions on Mars, which are 600,000 parts per million, or 60% water by weight." I was searching for information on moon rocks. It appears that they do contain considerable amounts of oxygen and my guess is that this is what accounts for much of the un-modelled absorptions. Does anyone still think that there would be any liquid water at 230C in a vacuum? IT will be interesting to learn how NASA managed to produce an estimate for the amount of water in the plume with only one experiement, no reference chemistry and only a guess for the particle size and thus thus the average photon path length! Best wishes, Tony | ||
Howard Mark (hlmark) Senior Member Username: hlmark Post Number: 294 Registered: 9-2001 |
Karl - I have to agree in principle with what you say, but then I also have to ask the question: how small does an absorbance have to get before it's effectively zero? When I did UV/Visible analysis in school, the "standard" pathlength for the UV/Vis measurements was 1 cm. Obviously, there was no noticeable absorbtion in the visible region from the water, at that pathlength. In fact, if you look through a drinking glass of water (approx 10 cm) there's still no noticeable absorption. And if you look into a swimming pool (a couple of tens of meters, perhaps) before the chlorine is added, or pond (if it's clear enough), there's still no noticeable absorption. Yes, if you look into the sea, then when the water is clear enough to see for an appreciable fraction of a kilometer, then it looks blue because of the absorption of the red light. So that gives us some sort of a benchmark. And while I don't know if anyone has actually measured the water absorption in swimming pool-sized pathlengths, the visible evidence is against there being any noticeable amount. But that's all in the visible; how much lower do the molecular absorptions get when you go into the UV? And so while in principle I have to agree with you, in practice I have to conclude not. The absorption mechanism for UV light has to do with the electrons, and is many orders of magnitude larger than the residual molecular absorptions. In fact, what every chemist "knew" back then was that when you got into the spectral regions where there might be some NIR absorbtion, they were always thought to be "too weak" to observe. Which was true for the instruments and methods and available pathlengths of the time. So I guess that statements like this are holdovers from that scientific upbringing. But to the extent that nobody has the luxury of a cell with kilometer-size pathlengths, we have to admit that in practice it's still true at least in the laboratory, even in most of the visible region, much less in the UV, I think. \o/ /_\ | ||
Karl Norris (knnirs) Intermediate Member Username: knnirs Post Number: 16 Registered: 8-2009 |
Howard, I don't agree with your statement that water is transparent down to 200 nm, if by transparent you mean no absorption in the region from 200 to 700 nm. You are ignoring the high-order overtones of the infrared bands. They will be very weak, but they will be there. Has anybody measured the visible spectrum of water at a pathlength of 100 cm or even 10 cm? Back to water on the moon. I obtain an insight to Dr. Colaprete's thinking from this web site: http://www.nasa.gov/multimedia/podcasting/arc-colaprete-lcross-20090401.html My spectra are intended to be a simulation of the emission spectra from the plume of dust, and broken rocks, with possible absorption from water as gas, liquid, or ice. My spectra are in linear energy format. I assume NASA measured the average temperature of the plume, and they mention a blackbody temperature of 230 C. There will be a lot of scatter from the particles in the plume and this should increase the pathlength for the water absorption. Karl | ||
Howard Mark (hlmark) Senior Member Username: hlmark Post Number: 293 Registered: 9-2001 |
Art - you're asking the wrong crowd a question like that!! Good thing at least one of us knows something beyond NIR. I know that liquid water is transparent to at least 200 nm; I recall that from my old Instrumental Analysis courses. At shorter wavelengths it may very well absorb, as Lois said, or, since UV interacts with the molecule's electron clouds, at even shorter wavelengths it may dissociate, and the products (neutral H, neutral OH, H+ and/or OH-. Conceivably even H-, although that's not too likely; probably spit out the extra electron immediately.) could absorb UV, as we discussed a little while back. Whoops: H+ has no electrons to interact with the UV, cancel that one! \o/ /_\ | ||
Lois Weyer (lois_weyer) Senior Member Username: lois_weyer Post Number: 31 Registered: 7-2002 |
Water is supposed to have an n to pi-star transition at 167 nm. | ||
Art Springsteen (artspring) Intermediate Member Username: artspring Post Number: 20 Registered: 2-2003 |
Hello again,all Like Howard, I got the OO flyer in the mail this morning. Good to see that NASA is supporting the American spectroscopic industry. although the fact that OO is opening a large plant in China is not good news. The question I have is where does water absorb in the UV (as far as I know, it doesn't...). So what was the OO spectrometer REALLY looking at? | ||
Howard Mark (hlmark) Senior Member Username: hlmark Post Number: 292 Registered: 9-2001 |
Two things here. First, which I was going to mention to Art anyway, was this: David Day must have some contacts at NASA. Maybe you could ask him to request one of his contacts to sign on to the discussion group and give us whatever information he can "from the horse's mouth" so to speak. Secondly, when I downloaded my e-mail this morning, there was a routine newsletter from Ocean Optics talking about THEIR spectrometer on the LCROSS mission! Here's the text of that article in the newsletter: "SPECIAL REPORT: Our Journey to the Moon Aboard the LCROSS Mission When NASA's LCROSS/LRO mission took off from Cape Canaveral, Florida on June 18, it carried with it a modified visible spectrometer setup from Ocean Optics and Aurora Design and Technology as part of its scientific payload. Named ALICE, the finished unit included our QE65000 Spectrometer and was to be used to measure lunar grain properties and H2O vapor to help NASA determine the presence of water on the Moon. On October 9, the mission send a Centaur rocket crashing into the Moon's surface in order to create a large plume of ejecta. That ejecta (debris) was what NASA's team would use to determine whether or not water was part of the Moon's composition. LCROSS's instruments successfully captured each phase of the impact sequence and the creation of the Centaur crater. NASA scientists announced Friday (November 13) that the signature of water was seen in both near-infrared and ultraviolet spectroscopic measurements taken during the mission. Ocean Optics' ALICE provided the ultraviolet measurements, confirming the findings of the near-infrared spectroscopic measurements. From the data gathered, NASA scientists were able to estimate that roughly 220 pounds of water were found by the instruments in the material excavated from the 20-30 m wide crater form by the Centaur impact. Ocean Optics is proud to have been a part of this historic mission and we look forward to our next space mission to Mars." | ||
Art Springsteen (artspring) Intermediate Member Username: artspring Post Number: 19 Registered: 2-2003 |
Hello again, I forgot to add- the NASA spectrum is a transmittance spectrum, not absorbance. | ||
Art Springsteen (artspring) Intermediate Member Username: artspring Post Number: 18 Registered: 2-2003 |
Hello all, Karl's analysis was quite interesting, but I had a chance to talk with David Day of Polychromix this afternoon. He will comment in full once his membership in this group is approved. Actually, the sun WAS used as the source. The satellite hit at essentially the south pole of the moon and the spectra of the sun was compared pre- and post impact, and the spectra was generated as the difference between the solar spectrum and the solar spectrum going through the plume (I think I have that right- David will certainly correct me!). There is a lot David cannot reveal due to Polychromix agreement with NASA but his explanation was simple but elegant. | ||
Karl Norris (knnirs) Member Username: knnirs Post Number: 15 Registered: 8-2009 |
I�m back, I am guessing on much of my observations, because I do not have the data and the details about the experiment. I have done a lot of Web searching, and found information to help support my guesses. The sun was not involved in this experiment, because NASA chose the particular crater as one that is not exposed to the sun, which could melt the ice and cause evaporation over time.. This choice was to maximize the chance of hitting ice with their rocket blast. The available radiation is from the heat generated in the rocket blast. NASA measured the temperature of the generated plume, and they show a smooth red line as the spectrum of a blackbody at 230 C. The measured radiation should be the blackbody radiation of the plume with an average temperature of 230 C., including the absorption of the different materials in the plume. If there was ice in that crater there should be water in different forms in the plume, and the NIR spectrum should show the loss of energy at the wavelengths where water absorbs. They hoped to find the water vapor bands in the 1340-1430 and 1800-1940 nm regions. However, the Polychromix spectrometer with a spectral bandwidth of 10 nm and a data-point spacing of 12.5 nm is not a good choice for detecting the very narrow bands of water vapor. The NIR spectrum from the NASA LCROSS web page shows a lot of character, with a significant amount of noise. To obtain a more complete understanding of the NASA data, I transferred their spectrum into my computer, and added a transmission spectrum of water vapor, and liquid water. The resulting spectra are attached. In transferring the LCROSS spectrum I discovered that the data spacing was 13 nm rather than the normal 12.5 nm spacing from the Polychromix literature, but this spectrometer may have been a special design for NASA. The spectra that I added are scaled to fit the graph, and represent unknown amounts of samples. I did not include the spectrum of ice, but it would be similar to that of liquid water with the two bands shifted about 50 nm to longer wavelengths. I observe some fit between the liquid water spectrum and the NASA spectrum, but the fit is not very good. I am willing to offer this speculation, because at my age this will not help or hurt my career. Karl 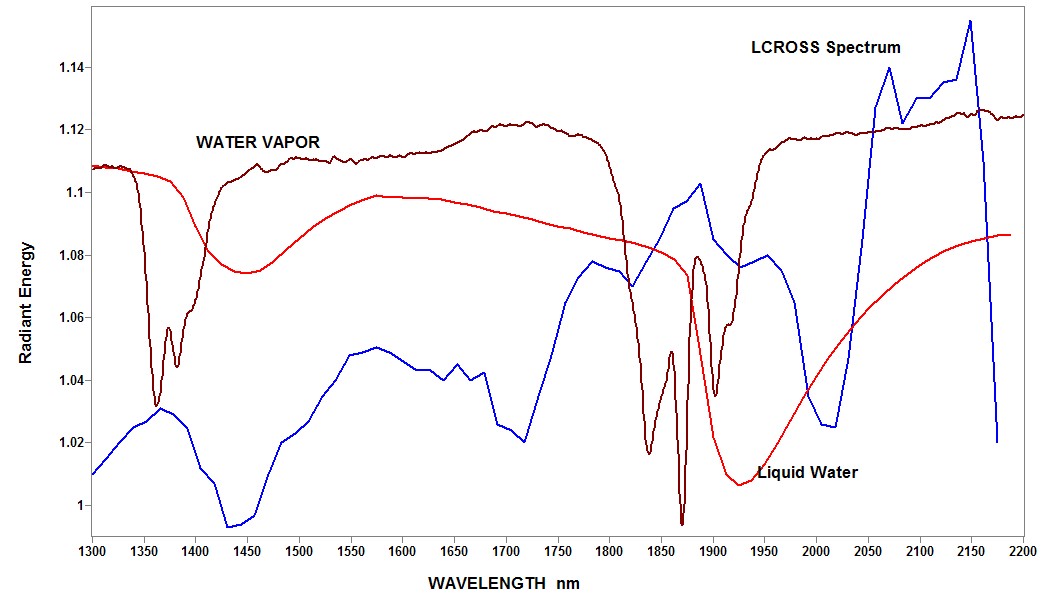 | ||
Howard Mark (hlmark) Senior Member Username: hlmark Post Number: 291 Registered: 9-2001 |
Tony - press releases are for the purpose of IMPRESSING the publid, not edifyng them!! But I disagree with your assessment: I'll bet that the NASA guys ARE reading these posts, and laughing their heads off!! But reflected sunlight would have the same distribution (or nearly so, at least, modified by the properties of the reflector. If the reflector was gray or nearly so, then the distribution would be that of the 10,000-degree blackbody, except at a lower energy level) as the sunlight itself. The total energy output from an actual blackbody radiator increases as the fourth power of the absolute temperature of the blackbody radiator. A blackbody at 230 deg C would be at 230+273 = 503 deg K, so compute the ratio of 503 / 273 or whatever reference temperature you want to use, and raise that to the fourth power. But there isn't an actual blackbody involved, only some energy with that same distribution of radiation, so the amount of power available could be anything. \o/ /_\ | ||
Tony Davies (td) Moderator Username: td Post Number: 215 Registered: 1-2001 |
Hi Howard, Not only would the general public NOT be able to understand the NASA spectra, few scientists would have any idea what it showed; few spectroscopist would want to guess at what it was showing. So the only people who can hope to have any comprehension of the spectra are a tiny group of NIR spectroscopists and we have to make so many guesses that we are probably all wrong (sorry leave Karl out of that group). Hi Karl, How much NIR energy do you get from a blackbody at 230C? Where is this blackbody? Is it sunlight reflected from the moon's surface? How do you avoid the sunlight? Is this all happening inside the crater? But the crater is dark and very cold! I am getting more and more confused! Hi Art and Don, None of these were my dollars so I mustn't comment! I hope we will be given answer to some of our questions but I guess (another one) that if anyone at NASA is alerted to this site they will not be too happy about us! Best wishes, Tony | ||
Howard Mark (hlmark) Senior Member Username: hlmark Post Number: 290 Registered: 9-2001 |
I think Karl and Art are saying the same thing, and I agree with Art's assessment: what we are seeing and commenting on is a press release intended for the general public, not a scientific paper with all the details we would expect in one. The general public would not understand that, anyway. \o/ /_\ | ||
Karl Norris (knnirs) Member Username: knnirs Post Number: 14 Registered: 8-2009 |
I agree with Dr. Dahm. I don�t understand why NASA provided such a poor spectrum on the Web, and then made claims which in my opinion can not be supported. I copied the following from a Web page of Polychromix. �NIR2007 EMERGING NIR TECHNOLOGIES presented by Dan Klevisha, Polychromix NASA LCROSS Project The mission involves using the upper stage of the space vehicle as a kinetic impactor to generate a cloud of dust. After launching the LRO satellite,the Shepherding Spacecraft (SSC) and the Centaur Upper Stage will remain coupled and will adjust their orbit. The SSC contains the LCROSS probe contains 2 POD NIR spectrometer interfaces to space telescopes. This is the ultimate example of on-site NIR or taking the analyzer to the samples as the mission will send 2 Polychromix POD MEMS NIR systems to help determine if there is water hidden in the permanently dark craters of one of the Moon�s poles. If there are substantial amounts of water ice there, it could be used by astronauts to make rocket fuel when they later visit the Moon. One of the LCROSS POD spectrometer/telescopes will be positioned to measure light which is reflected from the dust cloud using the sun as a source. The other is positioned to look outward using the sun to measure the dust in transmittance or diffuse transmittance if you prefer.� From this it appears that Polychromix expected NASA to use the sun as the source, and measure the absorption spectrum of the plume to show the presence of water. This was two years ago, and I assume that tests at NASA indicated that this procedure was not the optimum procedure, and that a measure of the emission spectra was a better procedure. I may be wrong, but I interpret the NASA LCROSS spectrum to be an emission spectrum with a blackbody source at 230 C. This is a satisfactory procedure, except that the spectral bandwidth of the spectrophotometer is 10 nm, and the data point spacing is 12.5 nm. This is not a good choice for measuring the rotational bands of water vapor, which have a bandwidth of less than 2 nm. The detection of liquid water or ice with these spectrophotometers is more realistic, because the spectra are quite broad. The text from NASA states they can detect water vapor and ice in the collected spectrum, but I am not convinced. I can accept the possibility that the spectrum in the 1450 nm region could indicate ice, but where is the spectrum of ice in the 1940 nm region. Please note that for ice the magnitude of the band in the 1940 nm region should be about three times larger than in the 1450 nm region. I look forward to more complete information from NASA on the detection of water vapor and ice from their experiment. | ||
Art Springsteen (artspring) Intermediate Member Username: artspring Post Number: 17 Registered: 2-2003 |
Hello Don and Tony, I wouldn't be so harsh on the media and NASA. The descriptions of the experiment certainly have to be 'dumbed down' for a general audience. We are certainly in the tiny minority who gives a rat's hindquarter how the measurements were made and have the technical knowledge to accurately assess their experimental data. That being said, it is frustrating to hear the claims ("24 pails of water') without really any evidence of how they came up with that figure. | ||
Donald J Dahm (djdahm) Senior Member Username: djdahm Post Number: 33 Registered: 2-2007 |
Well, Tony, it would help if I knew for sure what I was having �thoughts� on. Over the years, I have my share of disagreement with results published in the press. In an earlier post, I said that in this case, I found the reports vague and potentially misleading. I deserve better for my tax dollars. It is absolutely inexcusable, in my view, that we have to guess as to how the experiment was done, and how the data was collected, when the results have been touted so enthusiastically. Perhaps there is a website that describes this in detail, if I would just open my eyes, but I my cynicism hasn�t encouraged me to look for it. Having said that, I am glad that this has provided us with some amusement and the opportunity to examine how our techniques might fare in extreme conditions, and throw some light (pun intended)on things that we find exotic and interesting. However, if my assumptions are correct that we are looking at �black body� radiation which contains an absorption spectrum, the experiment is not being done in remission, but in transmission. Since we are dealing with a cloud, there is certainly scatter to worry about. I would say that the absorption signature of a material in such as case would be made to appear larger because of the scatter. I don�t dare to comment beyond that until I understand the experimental details better. | ||
Howard Mark (hlmark) Senior Member Username: hlmark Post Number: 289 Registered: 9-2001 |
Well, I guess David WON'T be at EAS, then! But as for Tony's question: aside from van der Waal's forces, the only reaction I can think of would be if UV bombardment of the surface knocked around some atoms and left surface free radicals, then those could react with the water, to form hydrides and/or surface -OH. Surface -OH is common on earth, all glass and quartz has that. My thesis advisor, Manfred Low of NYU, had done a lot of mid-IR measurement of surface -OH, to elucidate their chemistry. I probably have some old reprints of his which may have some info about the mid-IR spectrum, but I'm leaving for EAS now and won't be able to look them up until I come back, if there's still interest by then. I do recall that it all had to be done in vacuum, and that one of the major experimental problems was to get rid of the physisorbed water, which was much more plentiful and some of which was bound fairly tightly, so that only the -OH would be left to react. \o/ /_\ | ||
Art Springsteen (artspring) Intermediate Member Username: artspring Post Number: 16 Registered: 2-2003 |
Hello all, I was asked to get comments from David Day of Polychromix. David is on an island in Thailand with no web access but he did call Dr. Fred Haibach of Polychromix. Here is Fred's response and a transcript of David's phone reply. "Art, Dave is on vacation at the moment. It would be nice if Polychromix were to respond to the NASA announcement, but it would probably not contain any interpretation of the spectra or guidance on NASA designed components. That is wholly NASA's. It is public knowledge (although it is buried within the LCROSS website) that there were two standard Polychromix engines aboard LCROSS. One was forward-looking and the other side-ward. Reference spectra were taken of the sun, earth and sunlit moon during the transfer orbit and before impact. Both functioned properly until impact." From David Day "Hi Art, Please post this from me. I am on an island in Thailand with no electricity or hot water. I am borrowing someone's PC right now with something like a 9600 baud cell phone attached that gives me a painfully slow connection. I'll be connected later this week. Regards, David" Regards , Art | ||
Tony Davies (td) Moderator Username: td Post Number: 213 Registered: 1-2001 |
Hi, 1) Apologies for messing up the spacing with the over-large spectrum, cann't do anything about it until Ian returns from his post conference holiday! 2) I'm not very impressed with the NASA modelling; unless they have evidence for a strong emmission around 1,850 - 1,900nm. Otherwise if you get a positive peak when you add absorbances to a model, you have added too much! 3) Could the (unmodelled) absorptions around 1,600 - 1,800nm and 1,950 - 2050nm be due to OH-? They are quite similar to OH in alcohols but shifted to smaller wavelengths. I suggest this is the correct direction for the shift. As OH becomes more complexed it moves to larger wavelengths. However, I agree with Howard that we would have expected such bands to be quite sharp but then again this is probably a low resolution system. 4) Is there a possibility of some chemical reactions between liberated water and moon rock? This could give rise to the broad peaks. 5) Question for Don: What are your thoughts about the remission processes in this experiment? Best wishes to all, Tony | ||
Karl Norris (knnirs) Member Username: knnirs Post Number: 13 Registered: 8-2009 |
There is new information available at:http://www.nasa.gov/mission_pages/LCROSS/main/prelim_water_results.html The measured spectrum is not identical to the previous, and the red line has been replaced with a spectrum of their fit to water vapor and ice. This text is for the new spectra: "Data from the down-looking near-infrared spectrometer. The red curve shows how the spectra would look with water vapor and ice added in appropriate amounts to match the dips in the observations. The yellow areas indicate the water absorption bands. Credit: NASA" Karl | ||
Bruce H. Campbell (campclan) Moderator Username: campclan Post Number: 118 Registered: 4-2001 |
Interesting discussion. A little background information about the target can be found in ScienceDaily (Oct. 6, 2009). I found this article in Science Daily:news - a web site (www.sciencedaily.com). Basically the target was a polar one where sunlight never, or hardly ever, hits the surface. The temperature of the target was listed as "colder than minus 170 degrees Celsius." Also, the site had been earlier found to contain hydrogen. Hope this helps understand the news release and out discussion. | ||
Howard Mark (hlmark) Senior Member Username: hlmark Post Number: 288 Registered: 9-2001 |
Some miscellaneous comments: 1) The band around 1900 nm in Karl's spectra is showing the three "branches" (as they're called) of the vibration-rotation spectrum. The central line is the vibrational band, the outer two are the rotational substructures. It is clear, however, that the spectral resolution is far too broad to resolve the rotational lines, so all we can see is their envelope (I note that the three branches are barely separated from each other). 2) If all the H20 is broken up into H+ and OH- ions, then we'd expect no signal from the H+, but the OH- should exhibit absorbance due to the structure. I would expect the frequency to be shifted from that of neutral OH, but I don't know how much or in which direction. It should be a very sharp band, though, since I think the linear molecule wouldn't exhibit any rotational structure. There's also another possibility: that if the UV deposits enough energy into the molecule, then after the water breaks up, the OH- ion might be left with sufficient excess energy that it could actually emit radiation at the same frequency it would ordinarily absorb, and thus be a source of radiation at that frequency, rather than an absorber. Conceivably that could explain the change in direction of one of the bands. 3) In looking through the EAS preliminary program, I noticed that David Day is listed as a co-author on a paper being given at 9:00 AM on Thursday morning, "Detection of Mixture Components using Handheld NIR Spectroscopy" in a session entitled "Applications of NIR Spectroscopy". I'm attending EAS but I don't think I'll be able to get to that session. If anyone else will be there, and be available on Thursday morning to try to catch David (if he's there), maybe he'd be able to answer some of the questions we have. Could anyone volunteer to go there and report back to us? \o/ /_\ | ||
Tony Davies (td) Moderator Username: td Post Number: 212 Registered: 1-2001 |
Hi, There is more information about the project at: http://www.nasa.gov/mission_pages/LCROSS/searchforwater/LCROSS_impact.html They talk about water-ice but also water of hydration. They expected water vapour to be "broken" into H+ and OH- by UV from the sun. So what would we expect to see in the NIR spectrum? Tony | ||
Karl Norris (knnirs) Member Username: knnirs Post Number: 12 Registered: 8-2009 |
Tony Thanks for showing my spectra, and please note that the match with the NASA spectra is very poor for both water vapor and liquid water. If my assumptions are correct the presence of an absorber will be downward on the NASA spectra. Please note that the absorptions of water vapor and liquid water are much stronger in the 1850-1950 nm region than in the 1350-1450 nm region. Karl | ||
Tony Davies (td) Moderator Username: td Post Number: 211 Registered: 1-2001 |
Sorry that is a bit too large, hope this is better! Tony 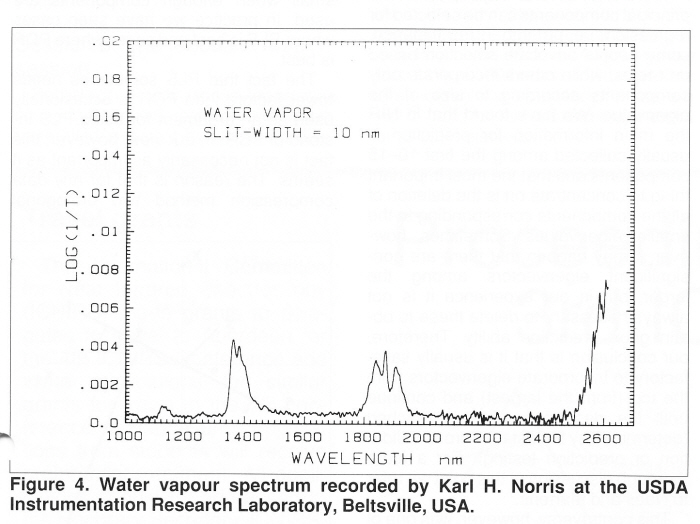 | ||
Tony Davies (td) Moderator Username: td Post Number: 210 Registered: 1-2001 |
Hi Guys! Questions � Questions � Questions Yes, Howard I have been interested in water vapour for some time! I thought it might be useful to put-up Karl�s spectrum of water vapour that he ran for me in 1985. You will see the shifts of about 60nm in the spectrum compared to the normal liquid water spectrum. Thanks Karl (for the spectrum again and reading the text). If the background temperature is 230C then can we assume that we have to be dealing with just water vapour? Illumination I had assumed that the plume would emerge from the crater and then be illuminated by the sun. But I am still uncertain about where you get a reference. Is it just trial an error moving the blackbody radiation? David Day, Hello Art! If David Day does not want to take part in an open discussion (he can join the group today) could you ask him to have a discussion with Karl and let Karl report to us? While I agree with you that water of hydration is much more likely, I don�t remember NASA saying anything about water being chemically bonded. Would the energy cost of releasing it be worth while? �Buckets� of water sound like the real thing even if it is ice and presumably a long way underground. Karl, If these are energy spectra should absorptions show as downward peaks? Best wishes, Tony 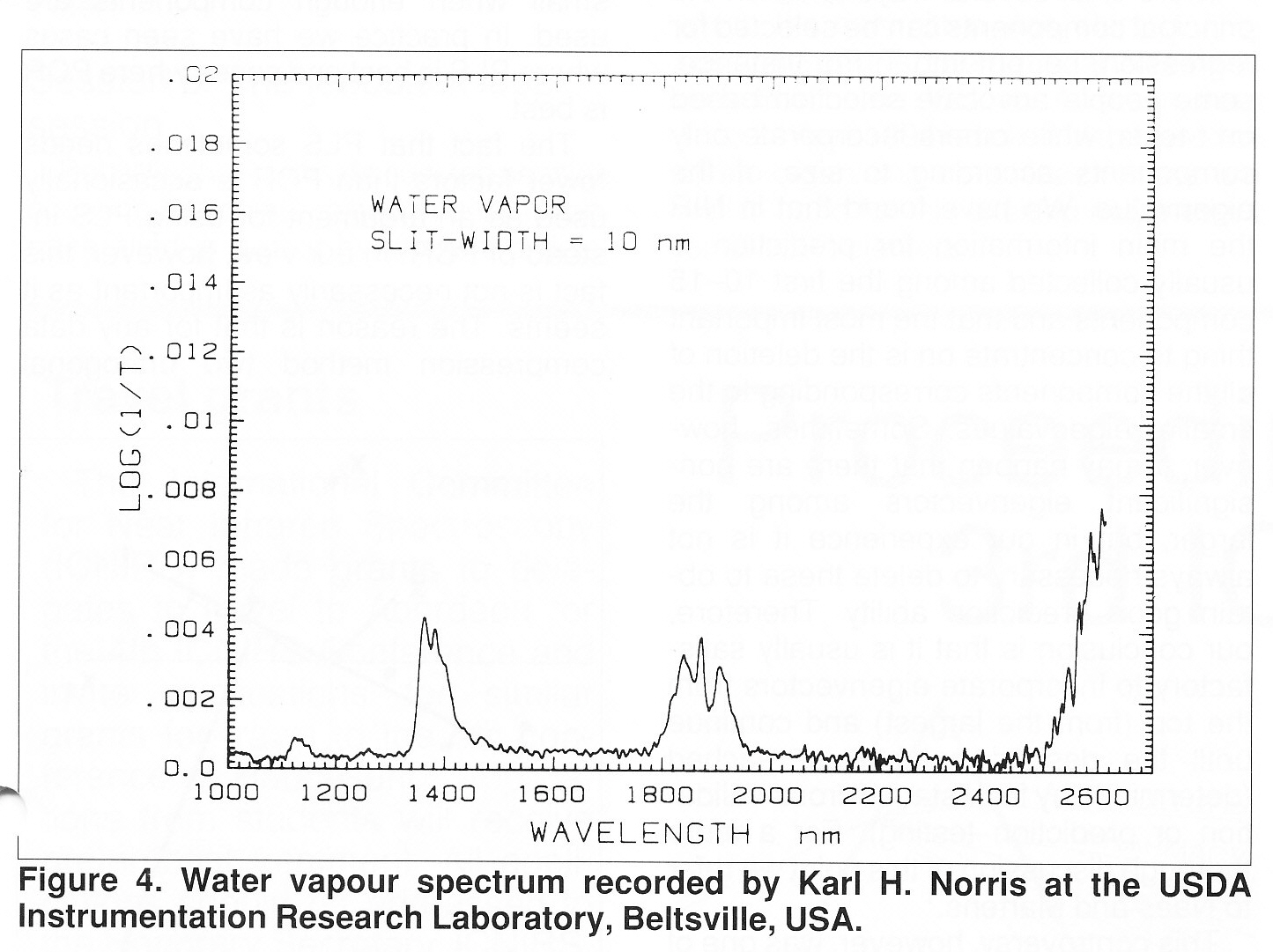 | ||
Karl Norris (knnirs) Member Username: knnirs Post Number: 11 Registered: 8-2009 |
Tony, The text with the spectral image states 230 C as the background temperature. Karl | ||
Howard Mark (hlmark) Senior Member Username: hlmark Post Number: 287 Registered: 9-2001 |
Art - David Day will be able to read all the postings on the discussion if he wants to, but unless he registers on the discussion site he won't be able to respond here. Therefore, if he responds to you directly, could you please post his comments here for us? \o/ /_\ | ||
Art Springsteen (artspring) Member Username: artspring Post Number: 15 Registered: 2-2003 |
Per Andrew McGlone's post: I know David Day pretty well. I don't think he follows this group, so I have forwarded the last three postings on to him. I'm hoping he will respond. Myself, I have little doubt that NASA found water, most likely entrained as water of hydration (it is everywhere, even under vacuum and low temperature), but I am rather skeptical about "24 gallons". Regards to all. Art | ||
Howard Mark (hlmark) Senior Member Username: hlmark Post Number: 286 Registered: 9-2001 |
Tony - A large part of the reason the bands of liquid water we observe are as wide as we see them is due to the interactions of the water molecules in the liquid with each other. This changes the energy levels of the individual molecules, leading to different molecules absorbing at different frequencies. This is true of most materials that can be measured as both liquid and gas. In the gas phase, where there are (almost) no interactions and the quantum levels are therefore the same for all molecules, what is normally observed in the gas (or vapor) phase is the rotational fine structure superimposed on the (single) frequency of the vibrational mode. You normally need fairly good spectral resolution in order to be able to separate the rotational lines from each other, but when you do, they're fairly sharp since their width is due only to Doppler effect and quantum uncertainty, as opposed to the liquid state where the interactions turn the spectrum into a continuum. Individual atoms or molecules flying around after the impact would almost never interact with each other, because they're all flying apart. Assuming that none of the atoms comes away from the impact with more than (the moon's) escape velocity, and since there's no air to interfere, each molecule would simply follow a ballistic path until the moon's gravity pulled it back down to the surface, just like the larger particles, such as the dust comprising the plume, that NASA took pictures of. Don't forget that a single molecule does not itself have a distribution of energies, nor, for the same reason, does it have a temperature (which is a measure of the AVERAGE energy of a collection of particles having a Boltzmann distribution - I remember THAT one very well because it was a question on one of the cum exams I took in grad school!). And when an individual molecule gets back to the surface, it would simply cling, by the usual chemical forces, to whichever particle (probably a dust particle) it happened to hit, again becoming part of the monolayer covering the surface. Karl makes a good point; it seems likely that the "background" curve itself may be what they used to determine the temperature. But now the question that just occured to me is: which temperature scale is that 230 degrees referred to: Celcius or Kelvin? Also, I think one of the things I said is almost self-contradictory: if the crater is cold because it is always in the dark and never gets any sunlight, then how could NASA use sunlight reflected off the crater floor as a reference (or for anything else, for that matter)? On the other hand, some of their pictures are purportedly of the crater floor before the impact, so then the question becomes where did they get the light used to take those pictures? The crater always being in the dark also puts a kibosh on possibilities of using reflected sunlight to measure the spectrum of the plume, or to take the pictures of the plume, either. So what did NASA use for their sources, to collect the pictures and the spectra of the whole process, pre- and post-impact? \o/ /_\ | ||
Andrew McGlone (mcglone) Intermediate Member Username: mcglone Post Number: 17 Registered: 2-2001 |
I spoke briefly with one David Day at the recent NIR2009 conference. He is with Polychromix and they supplied the NIR gear for the moon probe. It was pretty much the guts of their standard system that was used. I don't want to report what David told me, for fear of being inaccurate and not understanding vapour spectroscopy at all. Its probably enough to say that that he thought there was a 'water' signal there but also that he has only just got some of the data himself and hadn't really had a chance to work it through properly. Now he seemed a very friendly, enthusiastic and knowledgeable chap. Perhaps if someone knows him better, one of you americans, then they might persuade him to contribute something here? BTW David gave a great presentation at the conference. Showed a slide of one of his systems after it had gone under a forklift. All smashed up but the sensor and optics elements was still intact and worked perfectly when they put it into a new box. | ||
Karl Norris (knnirs) Junior Member Username: knnirs Post Number: 10 Registered: 8-2009 |
Hi Guys, I've enjoyed the speculation on the NASA data, and I wish to express my interpretation of the second spectral image. I interpret the smooth upward drifting red line to be the Blackbody spectrum for 230 degrees, and the measured spectrum is the energy spectrum of material in the blast. I assume they had a measurement of the temperature, and that it was 230 degrees. Karl Norris | ||
Tony Davies (td) Moderator Username: td Post Number: 209 Registered: 1-2001 |
Hi Howard, Yes, I thought that on the moon it is ice but it would be water vapour after the implosion. But what happens next? Single molecules of vapour cannot become ice, they just become very cold molecules. So the absorption spectrum should not change very much. It would be very interesting to know what model NASA has for the event. The plume must be quite hot or is it just warm? Any water (vapour) molecule colliding with a piece of moon would evapourate very rapidly if it were hot so there might not be time for many molecules to form ice. Standardisation: I guess they have an internal system in the spectrometer. As this is a crater that is permanenly dark there will be no background radiation from the moon, especially in the last few seconds of the descent (which ought to be the best spectra?). There are so many questions!! I was reminded that many years ago I tried to get spectra of thin layers of water. One spectrum I obtained had a very strong 1940 peak and almost no absorption in the 1400-1500 nm region. I did ask a few people what they thought and the consensus was that it was just an abberation but perhaps it is part of the spectrum from this experiment!! Best wishes, Tony | ||
Howard Mark (hlmark) Senior Member Username: hlmark Post Number: 285 Registered: 9-2001 |
Tony - under the conditions on the moon as we know them, liquid water couldn't exist. It could only exist as ice, vapor, or attached to a solid as surface water or water of hydration in any of it's various forms. A part of the moon in perpetual shadow would be very cold, and the vapor pressure of water would be very low. I used to have to know things like this, and the vapor pressure of water at dry ice temperature is roughly 1e-6 torr, a fairly decent vacuum itself. Therefore H2O present would have to be either ice or water of hydration; and even at 1e-6 torr, any ice would have evaporated (sublimed, actually) millions of years ago. Of course, it's probably a lot colder than that, but I don't know the numbers. Basically, that leaves only water of hydration as a viable possiblity. Of course, when the rocket hit it, the heat released would then vaporize whatever water was there, so it would be measured in the vapor phase. My guess for a reference is the reflected radiation from the moon's surface before the rocket hit. Before the rocket hit, it was also certainly possible for them to have measured the sun's radiation and then the reflected light from the moon's surface, as a way to get a good measure of the surface reflectivity. \o/ /_\ | ||
Lois Weyer (lois_weyer) Senior Member Username: lois_weyer Post Number: 30 Registered: 7-2002 |
Maybe the 1940 nm peak is showing band inversion due to saturation. We need to know more about the optics involved before criticizing. Also, the red curve in one of the documents is simply a calculated scattering curve ("gray light"). The red curve in the other document is labeled as a "model fit". It is only the caption on the web page that appears to be wrong, saying that the red curve is "gray light". In fact, the model fit shows some band inversion for the 1940 peak, so maybe they know that band inversion occurred. Let's see if someone at EAS will help clarify this for us. I believe there is a NASA speaker in one of the student seminars at least. | ||
Tony Davies (td) Moderator Username: td Post Number: 208 Registered: 1-2001 |
Hi, yes this is exciting but also confusing. Do we know what they are measuring on their absorption scale (I cannot read it). It is also not clear if they think they are dealing with liquid water or water vapour. If the area between 1800 and 1900 is measuring absorption (as up) then this would be correct for combination bands in water vapour. The corresponding area for the first overtone region is 1300 - 1400.This does also appear in the spectra. However, there is quite an amount of absorption at 1940 which would suggest the presence of liquid water but there is no corresponding absorption around 14-1500?? It would be interesting to know how these measurements are made. I have assumed that they rely on sunlight scattered by the dust cloud but where does the reference come from? Tony | ||
Donald J Dahm (djdahm) Senior Member Username: djdahm Post Number: 32 Registered: 2-2007 |
As I understand it, there had been some "buzz" about the fact that the mission was a complete bust, and they needed to get some results out that would get enough publicity to conteract that. I found some of the statements they published to be somewhat vague, and perhaps even misleading, if they were interpreted as results in a scientific report. I suspect they intentionally went to the side of over-optimism. I saw one guy holding a bucket when he was being interviewed, and he was saying how many buckets full of water the cloud contained. | ||
Howard Mark (hlmark) Senior Member Username: hlmark Post Number: 284 Registered: 9-2001 |
Jerry - yes, thanks for finding this and letting us know. I also find the spectra (at least, the NIR spectra. I don't know enough about how the UV spectra should behave to have an opinion) less than convincing. But one thing I note is that the red "reference" curve for the 'gray or "colorless" warm (230 C) dust cloud' is different in the two documents. Also, in the press release, the two regions, around 1450 nm and 1940 nm have their convexity in different directions, at 1450 it's convex downward while at 1940 it's convex upward. On the other hand, I can't really fault them for "dropping a bomb" as you call it. In the wider context it's called "marketing"; like everyone else NASA has to justify their existence, and a little self-promotion is not unreasonable. On the other hand, if they really did find water after all, that can be pretty exciting news. But I suppose they could have waited until they had more convincing results. \o/ /_\ | ||
iyas (iyas) Senior Member Username: iyas Post Number: 30 Registered: 7-2007 |
dear jerry jin thanks for this interesting news regards iyas | ||
Jerry Jin (jcg2000) Advanced Member Username: jcg2000 Post Number: 21 Registered: 1-2009 |
Hi, guys You probably know this big news from NASA. Rather than expressing my willingness to colonize the moon, I am more interested in how NASA's analyzers discovered water on moon. According to the following link I found in NASA's website, UV/Visible and NIR spectrometer was used in the discovery. More interestingly, a few spectra was also released by NASA to support its water discovery. http://www.nasa.gov/mission_pages/LCROSS/main/prelim_water_results.html I would have a lot of questions to ask if I were present in NASA's media conference. The association of the alleged hydroxyl(-OH) band with water is not convincing at present. Once again, NASA can't wait to throw out a big bomb to catch everyone's eyeballs. You can see these spectra and NASA's annotations in the following link: http://www.nasa.gov/mission_pages/LCROSS/main/LCROSS_results_images.html Jerry Jin |