| Author | Message | ||
Alisha (agnosus) Intermediate Member Username: agnosus Post Number: 19 Registered: 1-2009 |
Dear Karl, I will investigate if this is feasible for the third party involved and will get back to you. Thanks. Alisha | ||
Karl Norris (knnirs) Senior Member Username: knnirs Post Number: 63 Registered: 8-2009 |
I am willing to suggest that the changes in long wavenumber region are not from NIR vibrational changes, but are from some interaction with the instrument. The bands at the other end of the spectra are probably from true NIR vibrations. Unfortunately your instrument limits the spectra for this region. It should be possible to test my theory, if you have a way of changing the incident energy. One possibility is to insert a polystyrene film somewhere between the lamp and the fiber bundle. Polystyrene has a weak absorber in the 1150 nm region, and the polystyrene bands should appear on your #1 reflection spectrum. If my theory is correct the band at 1150 nm will be lost in #2 and #3. The thickness of the poly film should be such that the band at 1150 nm can be observed on sample #1. Please let me know if you can do my tests. Karl | ||
Alisha (agnosus) Intermediate Member Username: agnosus Post Number: 18 Registered: 1-2009 |
Hi Tony, This is an epoxy resin reaction as far as I know. I don't have more info about the reaction. Here is a less cluttered image:  | ||
Tony Davies (td) Moderator Username: td Post Number: 279 Registered: 1-2001 |
Hello Alisha, I'm finding it difficult to see the time sequence with so many spectra. Could you show a spectrum with,say three, spectra. It looks as though something is changing. I assume this is a carbon based resin so we would expect to get some absorptions and some of these should change during curing. Best wishes, Tony | ||
Art Springsteen (artspring) Senior Member Username: artspring Post Number: 44 Registered: 2-2003 |
The developing color is a result of the reaction taking place. It shows up in the NIR because of the chemical changes going on. If you could monitor the reaction at around 450 nm (22200 wave numbers), you would see a concurrent development of an absorbance peak due to the amber(yellow) light being absorbed in the blue region of the spectrum. There are a number of other places in the visible region that you could also monitor this change (ie. increasing reflectance in the yellow region, around 550 nm (18200 cm^-1) | ||
thomas ricour (tricour) Advanced Member Username: tricour Post Number: 24 Registered: 2-2006 |
dear Alisha, I agree with Venkatarman concerning color. There are 2 major facts wich sustain a NIR spectrum (if pure reflexion is eliminated): matrix itself and functions which could absorb. For you purpose, i would insist in looking for a changing into the matrix: density, particules size,temperature, diffusion... | ||
venkatarman (venkynir) Senior Member Username: venkynir Post Number: 151 Registered: 3-2004 |
Hi Agnosus; Welcome to NIR work group. To my knowledge NIRS is immaterial of color.However if it is carbon content then there is a chance. What about temp and material property ?. Can you able to brief the process. | ||
Alisha (agnosus) Intermediate Member Username: agnosus Post Number: 17 Registered: 1-2009 |
Dear All, I am trying to understand the spectral behaviour of a resin curing reaction (image attached below). As the reaction proceeds a transition from liquid phase to solid happens (as indicated by drop in baseline). The shift to right in the high wavenumber region must be due to color change but I can't quite explain why it appears like this (the color is amber). Also I can't explain the shift to the left in the low wavenumber range and the sudden transition to sharp peaks!? A reflection probe was used to acquire these spectra and the reaction is happening in a cuvette. I will appreciate your comments? Thanks. Alisha 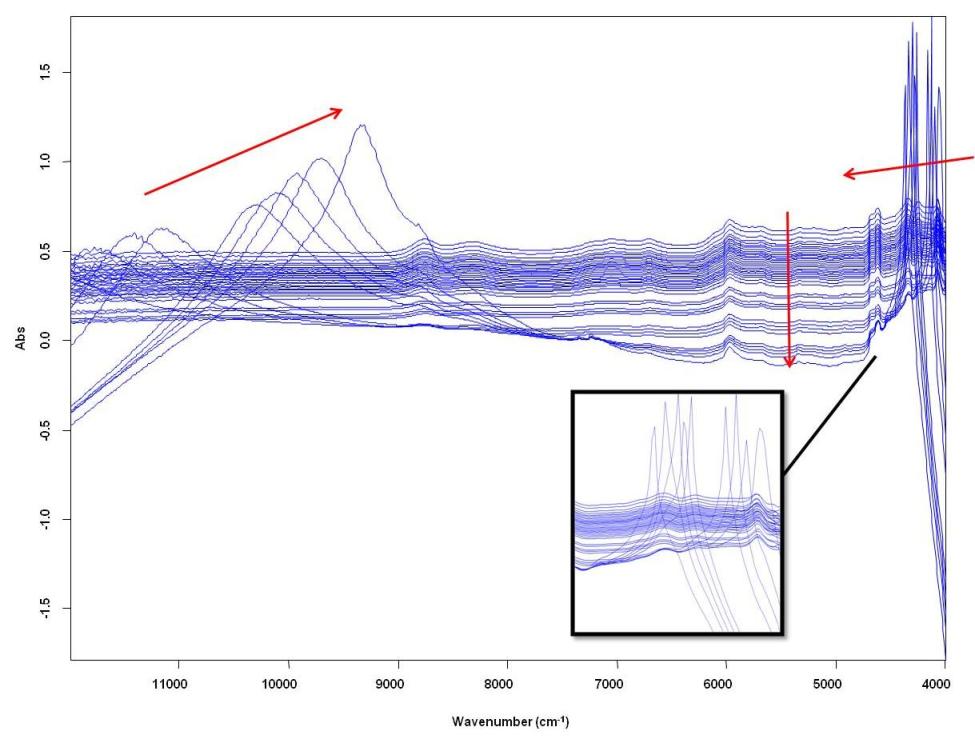 |